“These beautiful shots may look like works of modern art – but they are actually close-ups of chemical reactions. The works were snapped with the help of a group of scientists from the University of Science and Technology of China (USTC) in Anhui, China. Titled Beautiful Chemistry the project was the brainchild of Yan Liang, an associate professor at the university. Yan revealed he was fascinated by chemical reactions during his studies, so when he began working at USTC in February made a connection with two other professors. Supported by Tsinghua University Press and visual effects experts, Yan – along with the help of Professor Xiangang Tao and Dr. Wei Huang – researched which reactions may potentially look dazzling”. – Caters News

Cobalt chloride crystals growing in a solution of sodium silicate. (Photo by Yan Liang/Caters News)
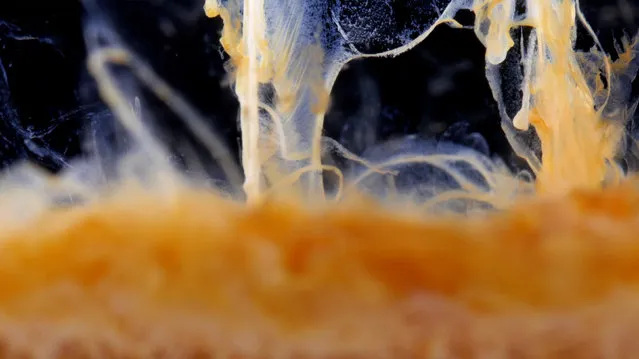
Silver thiosulfate precipitate (orange) formed after silver nitrate was added to a solution of sodium thiosulfate. (Photo by Yan Liang/Caters News)

Zinc reacting with copper sulphate solution to form copper (red). (Photo by Yan Liang/Caters News)

Magnesium reacting with acetic acid and generating hydrogen bubbles. (Photo by Yan Liang/Caters News)
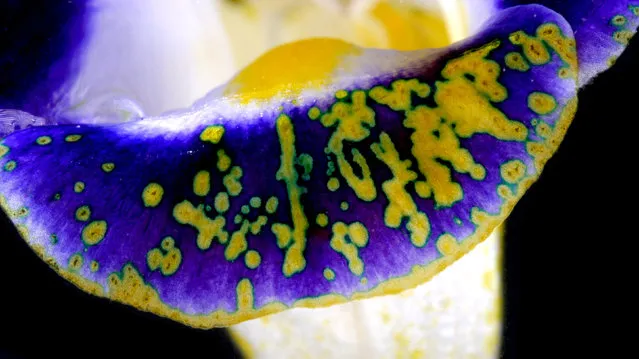
Wishbone (Torenia fournieri) flower that has had sodium hydroxide solution dropped on to its petal. Where the basic solution has hit, the petal has changed colour from purple to yellow. (Photo by Yan Liang/Caters News)

Basic copper sulfate precipitate (blue) formed after copper sulfate solution was added to a solution of sodium hydroxide. (Photo by Yan Liang/Caters News)

Silver chloride precipitate (white) formed after silver nitrate was added to a solution of sodium chloride. (Photo by Yan Liang/Caters News)

Purple cabbage being dipped into hydrochloric acid. The acid solution causes the cabbage to change colour from purple to red. (Photo by Yan Liang/Caters News)

Copper sulfate crystals. (Photo by Yan Liang/Caters News)
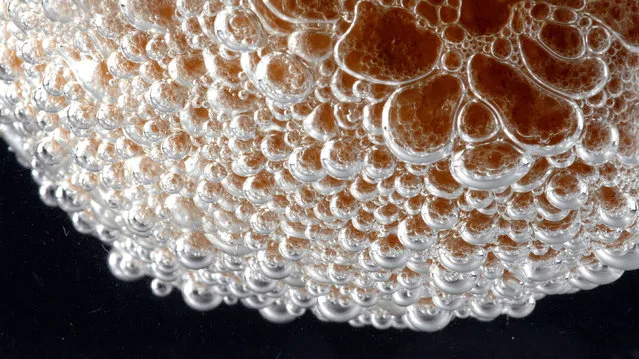
Eggshell reacting with hydrochloric acid to produce carbon dioxide bubbles. (Photo by Yan Liang/Caters News)
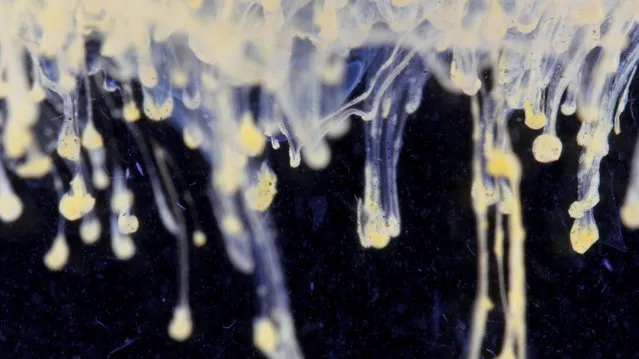
Cadmium sulfide precipitate (yellow) formed after csdmium nitrate was added to a solution of sodium sulfide. (Photo by Yan Liang/Caters News)

Nickel hydroxide precipitate (green) formed after nickel sulfate was added to a solution of sodium hydroxide. (Photo by Yan Liang/Caters News)
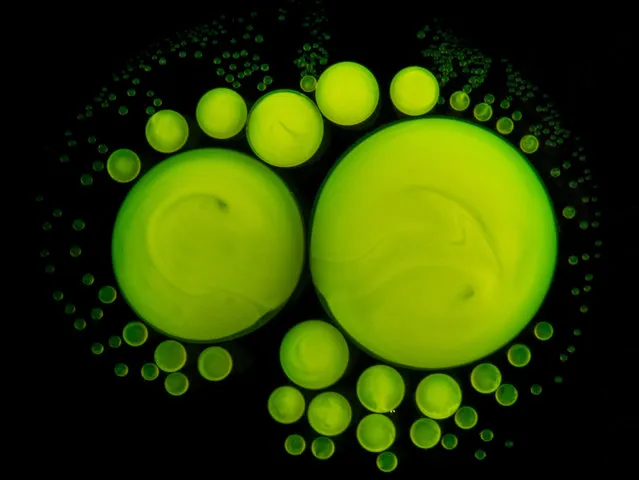
Green fluorescent droplets formed by mixing the chemicals inside a fluorescent stick. (Photo by Yan Liang/Caters News)
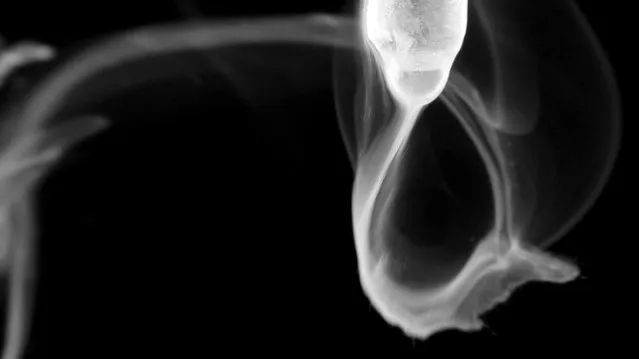
Ammonium chloride smoke formed from a reaction between hydrogen chloride gas and ammonia gas. (Photo by Yan Liang/Caters News)

Purple cabbage being dipped into sodium hydroxide solution. The basic solution causes the cabbage to change colour from purple to yellow. (Photo by Yan Liang/Caters News)

Zinc reacting with copper sulphate solution and sulfuric acid. The reaction with copper sulfate forms copper (reddish brown), while the reaction with sulfuric acid forms hydrogen bubbles. (Photo by Yan Liang/Caters News)

Zinc reacting with lead nitrate in a soft gel to form lead crystals. (Photo by Yan Liang/Caters News)
23 Oct 2014 11:33:00,
post received
0 comments
